Worthmed
Connecting healthcare innovators and MedTech for real-world testing
.webp)
.webp)
.webp)
.webp)
Worthmed’s goal was to create a collaborative platform to streamline clinical studies' design, deployment, and monitoring for MedTech developers, researchers, and healthcare providers. The aim was to reduce errors, accelerate validation processes, and simplify regulatory compliance for digital healthcare solutions.
The process of validating digital health solutions is time-consuming, complex, and error-prone, especially when aligning the needs of cross-functional teams of MedTech developers, researchers, and regulatory agencies. Worthmed needed a solution to centralize the design and monitoring of clinical studies while providing scalable and user-friendly tools.
Each study and research team has unique needs, so creating a streamlined yet dynamic user experience was a significant product, design, and engineering challenge.
— Bridging the gap between MedTech developers and researchers to design studies collaboratively.
— Ensuring the platform complied with national and European healthcare regulations.
— Building a user-centric platform for efficient clinical study design and monitoring.
— Maintaining interoperability and compliance throughout the process.
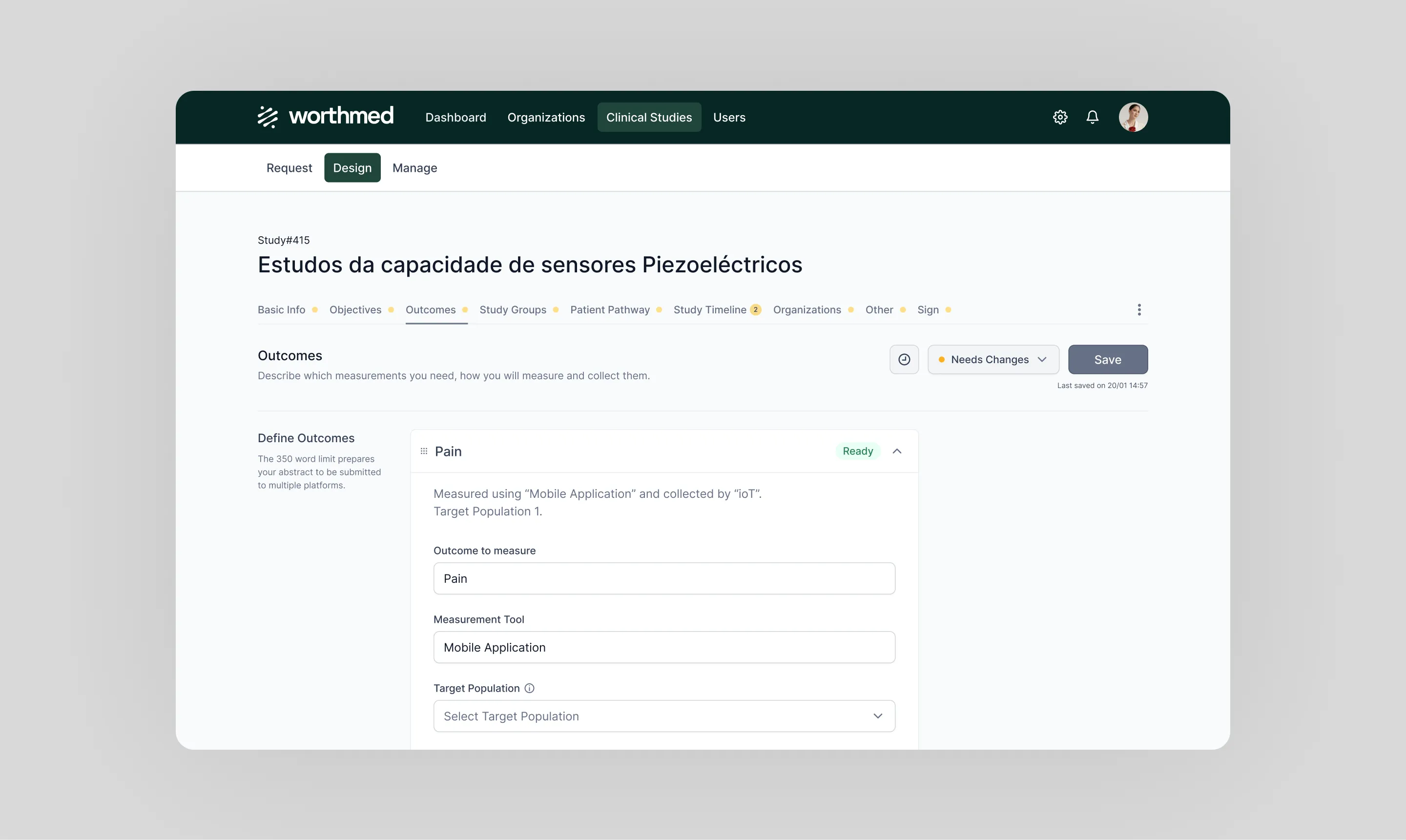
During the solution phase, Twistag conducted discovery sessions, workshops, and user experience (UX) interviews to ensure that the platform was built in alignment with the real needs of MedTech developers and healthcare researchers. These collaborative sessions let us gather stakeholder insights, identify key pain points, and shape the platform’s core functionalities.
Twistag developed an end-to-end multitenancy platform that empowered MedTech developers to collaborate with researchers on clinical study design, validation, and monitoring. The backend was developed using .NET Core, while the frontend leveraged Blazor WebAssembly (SPA), ensuring a seamless user experience and efficient data handling. This robust architecture enabled the platform to manage complex study workflows, allowing real-time collaboration between stakeholders and ensuring the secure storage and validation of critical clinical study data.
Our approach was based on hypothesis-driven development, aligning the platform with the healthcare sector’s needs.
Collaboration tools: MedTech developers and researchers can co-design clinical studies, review requests, and provide real-time feedback.
Data monitoring: Integrated tools allow secure, scalable storage and validation of clinical study data using AWS S3 and PostgreSQL.
Regulatory compliance: The platform supports data collection and presentation for approval by national and European medical authorities.
High performance: Utilized gRPC over REST to ensure efficient data transfer between services.
Interoperability: Leveraged FHIR standards to enable compatibility with external and third-party systems.
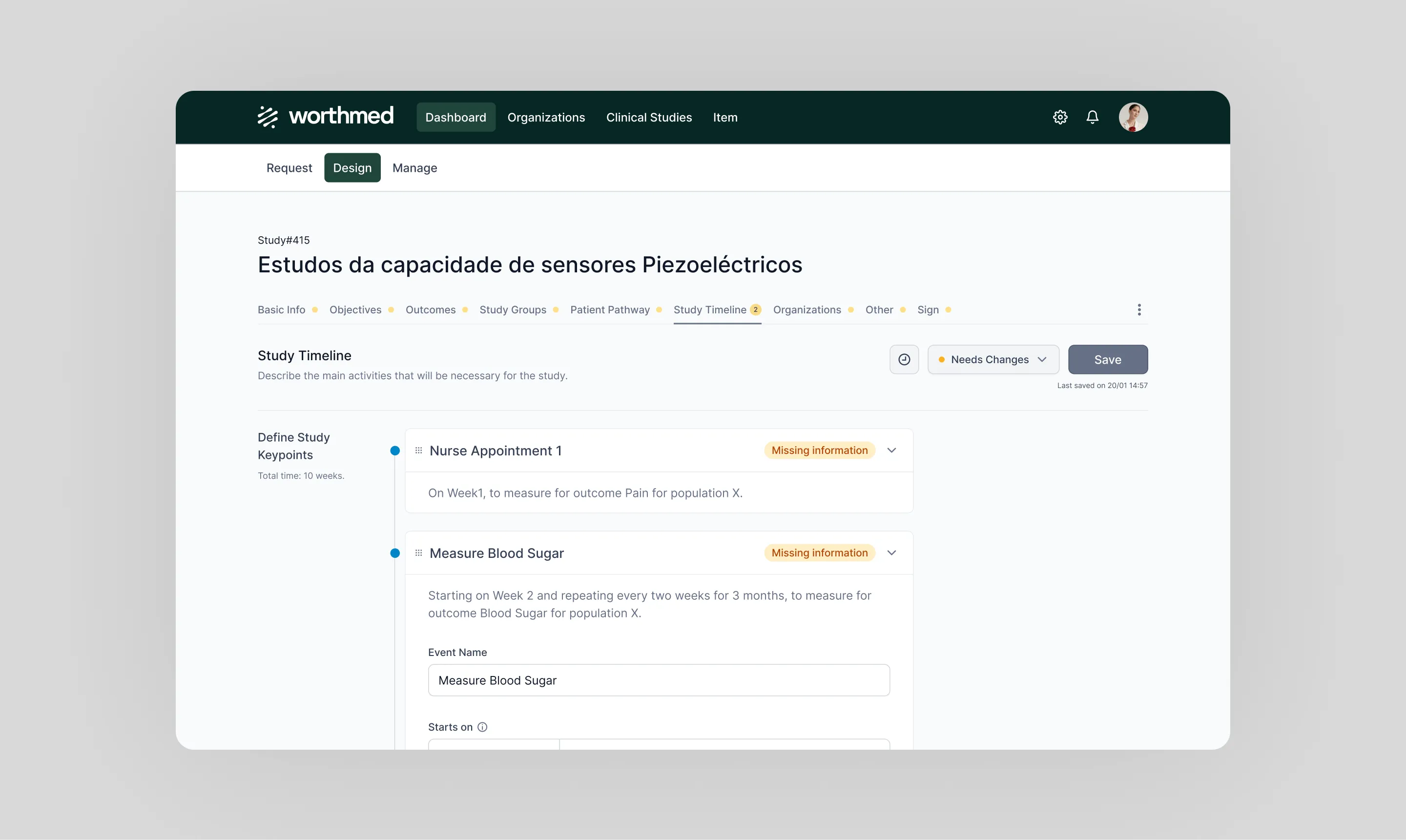
Twistag delivered a user-centric, scalable platform that significantly enhances collaboration, innovation, and validation for stakeholders in the healthcare sector. Currently onboarding multiple stakeholders, the platform has demonstrated impressive outcomes: clinical study design cycles are faster, effectively reducing the time required for MedTech companies to validate their solutions.
Additionally, the platform fosters seamless collaboration between MedTech developers and researchers while ensuring strict compliance with healthcare regulations, facilitating smoother approval processes with insurance companies and regulatory authorities.
Whether you need a dedicated team, end-to-end project, or a proof of concept, we're flexible to support your journey.
You’ll be speaking with one
of our tech project managers.